A Bowling Green State University research team led by Felix N. Castellano, Ph.D., is studying the photochemistry and photophysics of metal-organic chromophores. Currently, Castellano’s team is studying the photophysical properties of coordination and organometallic complexes of copper, platinum, ruthenium and iridium along with their potential application as photosensitizers for a variety of photochemical reactions.
“From the fundamental perspective, we are interested in the interplay of closely-lying excited states and the influence these interactions have on the resulting photophysics,” said Castellano, a professor of chemistry. “These processes are investigated with a battery of static and time-resolved spectroscopic techniques the latter revealing excited state dynamics evolving from the femtosecond regime to milliseconds.”
Castellano’s group uses the resources of the Ohio Supercomputer Center to better understand the nature of the static and time-resolved spectroscopic data measured for these molecules in the visible and infrared portions of the spectrum.
“The application of density functional theory (DFT) and time-dependent-DFT methods to the analysis of complex electronic structures of metal complexes has emerged as a powerful complement to optical spectroscopic measurements,” said Castellano. “These computational results are used in conjunction with experimental spectra to gain a deeper understanding of the electronic structure of the studied metal complexes than is possible with experimental data alone.”
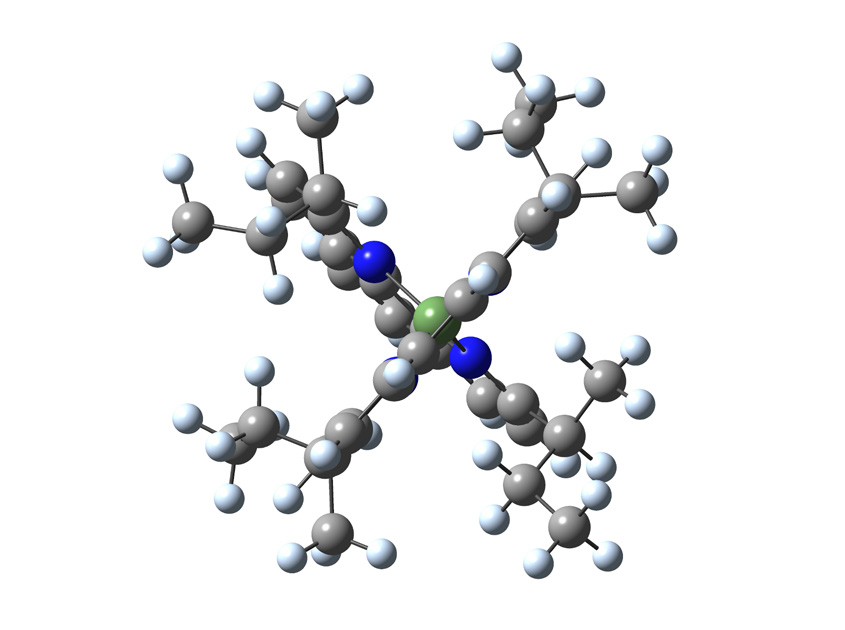
One focus of research in the Castellano group is the development of inexpensive copper containing dyes to replace more costly platinum, iridium and ruthenium sensitizers traditionally used in bimolecular photochemical reactions. The group currently is investigating a series of substituted copper bis-phenanthroline complexes for potential use as photosensitizers for photon up conversion. These types of copper complexes are known to undergo a large geometry change in the excited state leading to very short lifetimes.
These short lifetimes limit their usefulness as photosensitizers in chemically relevant bimolecular reactions. The researchers use DFT methods to compare the geometries of the complexes in their ground and lowest-energy excited state to predict which substituents will form complexes with the least amount of distortion in the excited state and, therefore, exhibit lengthened lifetimes. Calculations clearly demonstrate that increasing the size and number of aliphatic groups on the phenanthroline ligand decreases the amount of distortion observed in the lowest energy excited state.
--
Project lead: Felix N. Castellano, Bowling Green State University
Research title: DFT and TD-DFT on transition metal complexes
Funding source: Department of Energy, National Science Foundation
Web site: http://bit.ly/OSC-RR-Castellano