Akron team leverages supercomputers to better understand tie molecules
Columbus, Ohio (Oct. 5, 2011) –A special configuration of carbon atoms – a cylindrical network of molecules known as carbon nanotubes – is attracting a great deal of attention from industry researchers these days.
 |
 |
| Sadhan Jana, Ph.D. | Jie Feng, Ph.D. |
Carbon nanotubes (CNTs) can be applied as additives to various structural materials through a process called adsorption, where they are used to modify the surface of industrial materials in order to achieve certain properties, such as water repellent coatings for automobile windshields and hydrophilic coatings for contact lenses. This potential has drawn interest from industry researchers in many areas, such as aerospace/naval materials, nano-electrical products, optical devices, chemical sensors, catalyst supports, water/gas treatments, drug carriers and artificial tissues.
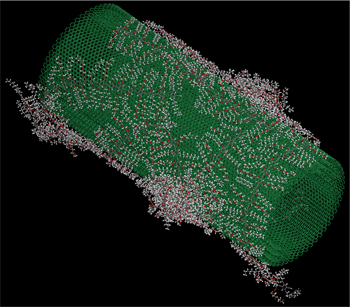
CNTs are composed of the same element as diamonds but with a different structural arrangement, and possess extraordinary thermal, mechanical and electrical properties. Individual nanotubes naturally align themselves into cylindrical “ropes” held together by van der Waals forces, the attraction forces found among atoms, molecules and surfaces and caused by correlations in the fluctuating polarity of other nearby particles.
Sadhan C. Jana, Ph.D., professor of Polymer Engineering at the University of Akron (UA), has been studying the interesting properties of these molecules by simulating these microscopic structures using the powerful systems of the Ohio Supercomputer Center (OSC).
“The biggest obstacle in realizing the full potential of CNTs is agglomerate formation owing to van der Waals and electrostatic interactions between individual CNT particles,” explained Jana. “Researchers have devised several methodologies to weaken such interactions.”
Two major approaches are followed in applying CNTs to material surfaces – covalent and non-covalent functionalization. In covalent functionalization, chemical bonds are formed with the surface carbon atoms, a process which often alters the graphitic characteristics of CNTs and compromises the electrical conductivity and mechanical strength of the molecule. In contrast, non-covalent functionalization utilizes uniquely designed tie molecules, a molecular segment that helps to improve the stability of CNTs by creating “ties” between the CNTs and polymer chains or solvent molecules to provide exceptional toughness, impact resistance and resistance to cracking.
“The simulations of polymer nanocomposites in solution are CPU-intense tasks,” said Jie Feng, a postdoctoral research fellow working with Jana at UA. “In our approach, the resolution of simulation is increased for the parts that are of utmost importance, for example, the phenomena at or near the nanotube surfaces, while low resolution is used for simulation of the parts of the system, such as the motion of solvent molecules.”
Jana and Feng conducted simulations of adhering tie molecules onto material surfaces and obtained estimates of improved mechanical properties and thermal conductivity. Their research focuses on gaining a fundamental understanding of the mechanism of physical transference – or “adsorption” – of such tie molecules from solutions onto surfaces of multi-walled carbon nanotubes (MWCNTs). The tie molecules may include polymers, surfactants or biopolymers. The CNTs treated with the tie molecules may be used in the fabrication of sensors and devices or may be compounded with the host polymers to create bulk polymer composites.
The Akron researchers are collaborating with experimentalists at a pair of Ohio-based companies, Zyvex Technologies and PolyOne Corporation, to conduct this research. The investigators believe their research will provide industry with guidance and theoretical explanations to aid in the development of tie molecules and value added composite materials for automotive, naval and aerospace industry applications.
“With the rich manufacturing history of this state, advanced materials is a natural fit for the staff and resources of the Ohio Supercomputer Center,” noted Ashok Krishnamurthy, interim co-executive director of OSC. “Dr. Jana’s carbon nanotube research is extremely well-suited for our systems and has great potential to help further the reputation of Ohio industry as one that competes on the leading edge.”
OSC systems are particularly well suited for industrial research applications. The center created the internationally recognized Blue Collar Computing program in 2004 to promote industry’s use of supercomputing. Access to powerful modeling, simulation and analysis resources provides companies with a competitive edge through improved manufacturing processes that can reduce the time, labor and cost needed to bring products to market. In fiscal year 2011, industry consumed nearly 1.5 million CPU hours on OSC’s flagship Glenn IBM 1350 Opteron cluster.
The Ohio Supercomputer Center (OSC) addresses the rising computational demands of academic and industrial research communities by providing a robust shared infrastructure and proven expertise in advanced modeling, simulation and analysis. OSC empowers scientists with the vital resources essential to make extraordinary discoveries and innovations, partners with businesses and industry to leverage computational science as a competitive force in the global knowledge economy, and leads efforts to equip the workforce with the key technology skills required to secure 21st century jobs. For more, visit www.osc.edu.
The Department of Polymer Engineering at the University of Akron is dedicated to providing the global society with leadership technology for a broad base of commercially viable polymer-related materials, processes and products through the development and application of our core competencies, which include education, research, analysis, modeling, design, synthesis and performance testing. For more, visit www.poly-eng.uakron.edu.