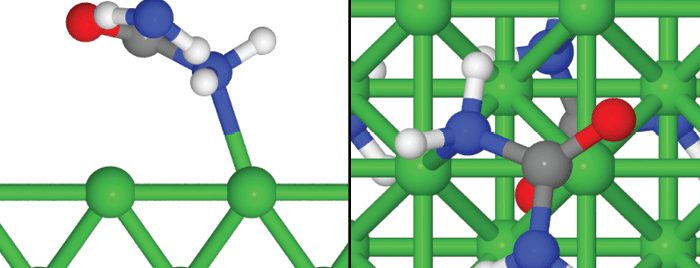
Simulations created by Gerardine Botte’s research group at Ohio University display a side (left) and top (right) view of a possible unit cell of monodentate urea adsorption on Ni(1 0 0). The red oxygen atom is in the top position (T), one blue nitrogen atom is in the hollow position (H) and the other blue nitrogen atom is close to the bridge position (B).
Coal is currently the largest source of electricity generation in the United States, while gasoline and diesel fuel power most vehicles. However, coal, gasoline and diesel fuel are non-renewable resources, and the combustion of these fossil fuels produces various pollutants. As a result, alternative, non-polluting energy sources such as hydrogen are desirable. Unfortunately, most hydrogen production processes also rely upon fossil fuels. Therefore, there is a clear need for renewable energy sources that can power hydrogen production, but at the same time mitigate pollution.
“Although electrolysis of water has been considered, a new technology to produce hydrogen by the electrochemical oxidation of urea from animals and humans is plausible and provides an opportunity to remediate waste,” said Gerardine Botte, Ph.D., Russ Professor of chemical and biomolecular engineering in the Russ College of Engineering and Technology at Ohio University. “Using nickel oxyhydroxide as the catalyst, hydrogen and nitrogen can be produced in a basic medium, and the process should consume 70 percent less energy than water electrolysis.”
In the presence of hydroxide ions, urea is electrochemically converted to nitrogen and hydrogen using nickel-based electrodes. However, the mechanism of this reaction has not been fully understood as the conversion of urea typically decreases over time. Botte’s research team has been investigating the interaction of urea and hydroxide ions with nickel as an initial study in understanding the conversion of urea.
Botte’s team is using atomic-level modeling to obtain a better picture of the surface chemistry during urea oxidation. The modeling is based on Density Functional Theory (DFT) as applied in CRYSTAL, a localized atomic basis set code, and comparisons are being made to in-situ measurements using X-ray Diffraction, Raman and Infrared spectroscopy methods at Ohio University’s Center for Electrochemical Engineering Research (CEER). Due to the large amount of parallel CPU time required for the calculations, Botte identified the need for supercomputing capabilities made available by the Ohio Supercomputer Center.
“We are performing geometry optimization and frequency calculations at varying adsorption geometries and coverage of urea and hydroxide on nickel using Crystal09,” explained Damilola Daramola, Ph.D., a multiscale-modeling scientist at CEER. “These results are providing information on the electronic, geometric and vibrational properties of the adsorbates and substrate and could help in furthering the knowledge of this urea-to-hydrogen technology.”
Project Lead: Gerardine Botte, Ohio University
Research Title: Computational studies of urea and hydroxide adsorption on nickel surfaces
Funding Source: Army Construction Engineering Research Laboratory
Website: http://www.ohio.edu/engineering/about/people/profiles.cfm?profile=64EA7FE7-5056-A800-489FB6C99184807