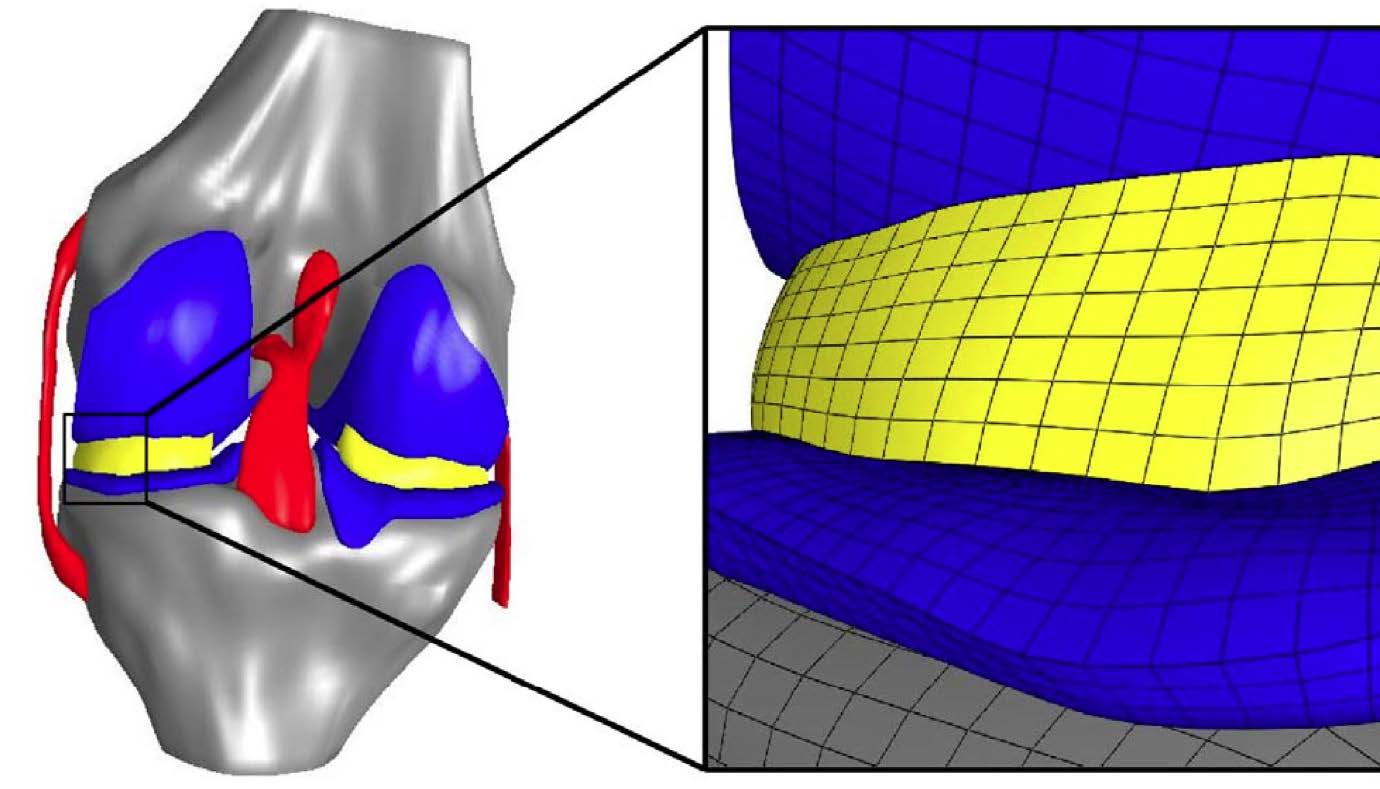
A finite element model of the knee joint with representation of the cartilage, menisci and the associated bone structures. The enlarged model region to the right illustrates the mesh resolution of the simulation.
A Cleveland Clinic research team is developing virtual models of human knee joints to better understand how tissues and their individual cells react to heavy loads – virtual models that someday can be used tounderstand damage caused by the aging process or by debilitating diseases, such as osteoarthritis.
Led by Ahmet Erdemir, Ph.D., the team is leveraging the powerful computing systems of the Ohio Supercomputer Center to develop state-of-the-art computational representations of the human body to understand how movement patterns and loads on the joints deform the surrounding tissues and cells. Erdemir is the director of the Computational Biomodeling Core at the Lerner Research Institute (LRI) in Cleveland, Ohio.
“The aging process and debilitating diseases affect many aspectsof the mechanical function of the human body, from the way we move to how our muscles, joints, tissues and cells accommodate the loading exerted on the body during daily activities,” Erdemir explained. “Computational modeling techniques provide an avenue to obtain additional insights about mechanics at various spatial scales.”
Many macro-scale studies have looked at how the various components of a knee joint – cartilage, menisci, ligaments and bone – respond to weight and other external loads. However, Erdemir and colleague Scott C. Sibole, a research engineer at LRI, wanted to better understand how those large mechanical forces correspond to the related deformation of individual cartilage cells – or chondrocytes – within the knee. Previous micro-scale studies of cartilage have not commonly been based on data from body-level scales or, in particular, on musculoskeletal mechanics of the knee joint.
In addition, calculated deformations typically have been for a single cell at the center of a 100-cubic-micrometer block of simulated tissue; Erdemir used an anatomically based representation that calculated deformations for 11 cells distributed within the same volume.
“In both micro-scale approaches, the cartilage cells experienced amplified deformations compared to those at the macro-scale, predicted by simulating the compression of tissues in the knee joint under the weight of the body,” Erdemir found. “In the 11-cell case, all cells experienced less deformation than the single cell case, and also exhibited a larger variance in deformation compared to other cells residing in the same block.”
Erdemir’s method proved to be highly scalable because of micro-scale model independence that allowed exploitation of distributed memory computing architecture.
--
Project lead: Ahmet Erdemir, The Cleveland Clinic
Research title: Chondrocyte deformations as a function of tibiofemoral joint loading predicted by a generalized high-throughput pipeline of multi-scale simulations
Funding source: National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health
Web site: http://bit.ly/OSC-RR-Erdemir